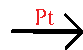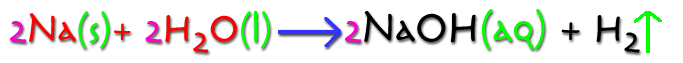|
A chemical equation is a short hand expression of a chemical
reaction. There are two parts to a chemical equation. The reactants
are the elements or compounds on the left
side of the arrow (-->). The elements and compounds to the right
of the arrow are the products.
Identify the reactants and the products in the equation below.
Reactants: 2 H2
+ O2 Look at the equation above. Notice the two purple 2s. These numbers are called coefficients. Coefficients are used to balance chemical equations. The blue arrow is a yield sign. It separates the reactants form the products. Most yield signs will be like the one above, but there are some other yield signs you need to know.
The following symbols are written in parenthesis after a formula and represent the state of the element or compound.
Identify the following about the equation below: the reactants, the products, the coefficients used, the state of each element or compound.
Reactants: 2 Na (s)
+ 2H2O (l) |